
The chromosphere, as seen in many eclipse images and video, appears intensely white in its lowermost layers and then has a characteristic purplish pink hue for most of its remaining extension. The reason is to be found on the type of light emitted by the elements forming it. So we need to look at the spectrum of solar light.
The spectrum of solar light is continuous under everyday circumstances. The photons originating from thermonuclear reactions inside the Sun slowly make their way to the surface and radiate away at all wavelengths (being visible to the naked eye between about 400nm and 700nm). While travelling through the solar atmosphere photons at specific wavelengths are absorbed by specific elements like Hydrogen, Magnesium, Sodium and Iron. These absorption phenomena create dark lines in the continuous spectrum and are the hallmarks of the presence of those elements in the solar atmosphere.
The spectrum of solar light changes rather dramatically just a handful of seconds before second contact of a total solar eclipse and reverts back to its normal state just a handful of seconds after third contact. The Figure displays an example of such a transition during the 2013 November 3rd annular-total eclipse. The usual intense continuous spectrum gives way, for some brief precious moments, to the mesmerising flash spectrum. When the Moon has almost entirely covered the photosphere, the intensity of the solar continuum plummets rapidly before vanishing and the much fainter light emitted by elements in the chromosphere and the inner corona comes into view. The continuous spectrum with dark absorption lines is replaced by a discrete emission spectrum with a few intense arcs and a myriad of fainter ones. As totality progresses and the Moon covers the chromosphere (at least as seen from around the centreline, unless the eclipse is intrinsically very short), these arcs fade away and only the faint and ghostly spectrum of the corona remains visible. This following images, captured during ATSE2013 by Kostas Emmanouilidis, shows the moments of this transition around the time of second contact. ,
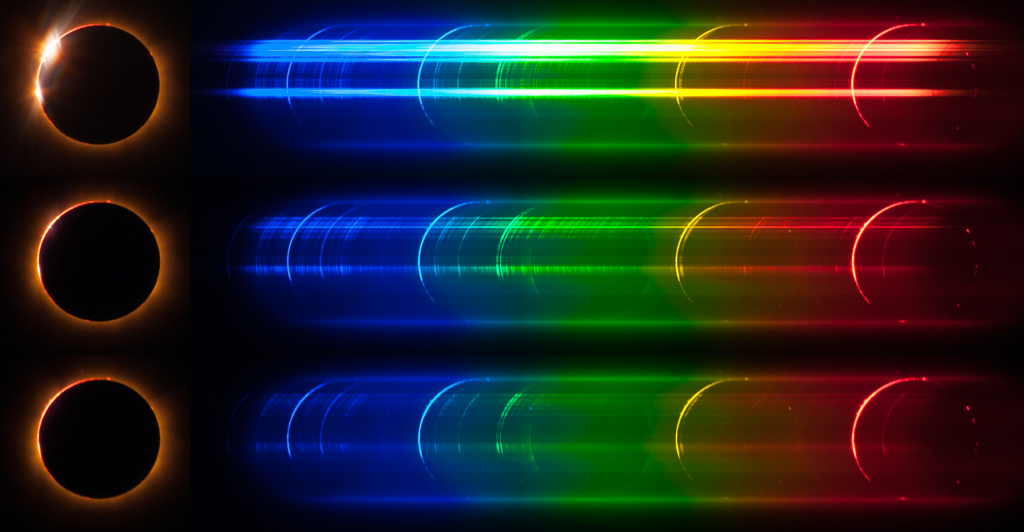
This Figure shows the origin of the main emission lines in the flash spectrum. Electronic transitions of various elements, in the neutral state or in ion form, emit photons at specific wavelengths. Prominent are the emission arcs of the Balmer series of Hydrogen (H-alpha,H-beta, H-gamma), some emission arcs from Helium, the Magnesium Triplet and the Sodium Doublet. The forest of faint arcs is due to a host of elements like Iron, Calcium, Barium, Titanium, and Chromium. Superposed on the discrete spectrum is the very faint, almost ghostly, circular-shaped diffuse spectrum of the inner corona. The following figure identifies some of the origin of some of the main lines that we can see in the flash spectrum.
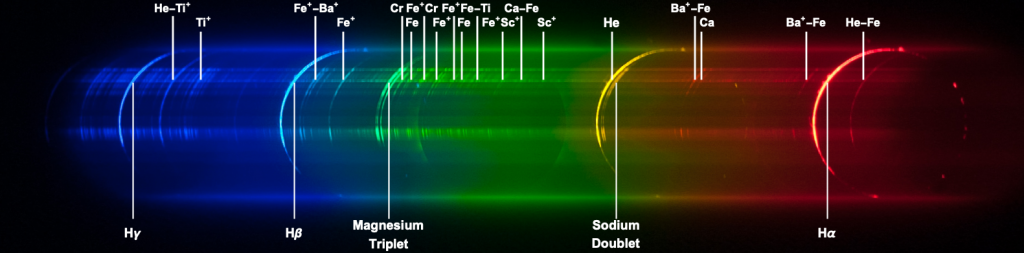
To provide a deeper explanation of the structure of the flash spectrum we need to make some assumptions on the distribution of elements in the chromosphere.
The Sun is not a solid body and the top of the chromosphere is turbulent and the place of occasional large prominences. Nevertheless, for simplicity’s sake we provide some approximate indications of where the light emitting elements are located. Hydrogen may be present, in probably abundant density, up to a height of ~12000 km above the surface of the photosphere, Helium up to ~8000 km, Magnesium up to ~3000 km, Sodium up to ~1500 km, and Iron and a host of other elements up to a height of ~1000 km. Given these heights, we can infer that, at second contact for example, a wider arc of light will be emitted by Hydrogen, extending outwards by at least 12000 km, than by Iron which extends outwards by only 1000 km.
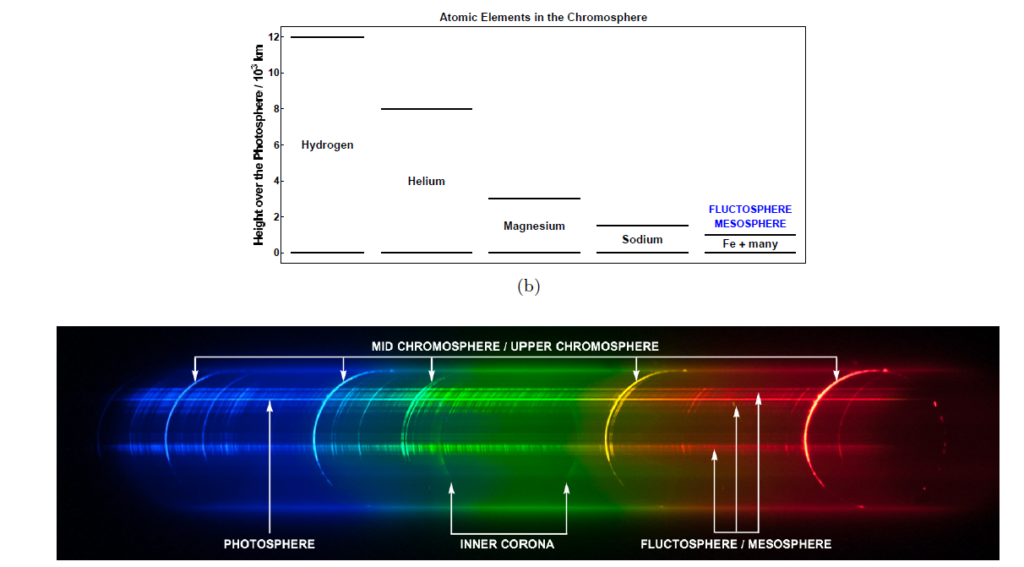
Armed with this knowledge we can try to explain the naked eye appearance of the chromosphere.
As most of the chromosphere above ~3000 km is dominated by the emission of Hydrogen and Helium, when we combine the respective red, yellow, aqua and blue emission lines, we get that deep purple-red colour that is so characteristic of many images of the chromosphere. However, much closer to the photosphere, besides the emissions of Hydrogen, Helium, Magnesium and Sodium, we have a myriad of emission lines by Iron and other elements. These lines are discrete but they are very densely distributed across most of the visible spectrum. The combined effect of all these emission lines is to generate a whitish light very close in aspect to the light emitted by the photosphere, distinctively different from the pinkish-purple one of the majority of the chromosphere lying higher up
The corresponding spectrum clearly explains the origin of such differences in colour. If we call mesosphere this lowest-lying layer that is the source of he myriad of faint emission lines which emits a quasi-continuum of white light, it is troublesome to disentangle the location of the very edge of the photosphere from the mesosphere in white light images.
The flash spectrum comes to the rescue as it allows, at least in principle, separation of the light coming from the photosphere, the fluctosphere, the rest of the chromosphere, and from the corona.
